Find stepby-step Chemistry solutions and your answer to the following textbook question: Consider these reactions, where M represents a generic metal. $$ \ce 2M (s) + 6HCl (aq)->2MCl3 (aq) + 3H2 (g), $\Delta H_1$ =−556.0 kJ $$ $$ \ce HCl (g)->HCl (aq) $\Delta H_2$ =−74.8 kJ $$ $$ \ce {H2 (g) + Cl2 (g)->2HCl (g) $\Delta H_3$ =−1845
Consider these reactions where m represents a generic metal – YouTube
Find stepby-step Chemistry solutions and your answer to the following textbook question: Consider these reactions, where M represents a generic metal 1.) $\ce 2M (s) + 6HCl (aq) \rarr 2MCl3 (aq) + 3H2 (g) \Delta H= -748.0 kJ $ 2.) $\ce HCl (g) \rarr HCl (aq) \Delta H= -74.8 kJ $ 3.) $\ce {H2 (g) + Cl2 (g) \rarr 2HCl (g) \Delta H= -1845 kJ

Source Image: weebrevues.com
Download Image
Chemistry Question Consider these reactions, where M represents a generic metal. 2M (s) + 6HCl (aq) \rightarrow → 2MCl _3 3 (aq) + 3H _2 2 (g) \hspace 1cm \Delta H_1 ΔH 1 = -600.0 kJ HCl (g) \rightarrow → HCl (aq) \hspace 3.9cm \Delta H_2 ΔH 2 = -74.8 kJ H _2 2 (g) + Cl _2 2 (g) \rightarrow → 2HCl (g) \hspace 2.9cm \Delta H_3 ΔH 3

Source Image: youtube.com
Download Image
Types of Chemical Reactions | Let’s Talk Science Question: Consider these reactions, where M represents a generic metal. 1. 2. 3. 4. 2M (s)+6HCl (aq) 2MCl3 (aq)+3H2 ( g)HCl (g) HCl (aq)H2 ( g)+Cl2 ( g) 2HCl (g)MCl3 ( s) MCl3 (aq)ΔH1=−607.0 kJΔH2=−74.8 kJΔH3=−1845.0 kJΔH4=−233.0 kJ Use the given information to determine the enthalpy of the reaction 2M (s)+3Cl2 ( g) 2MCl3 ( s) ΔH=
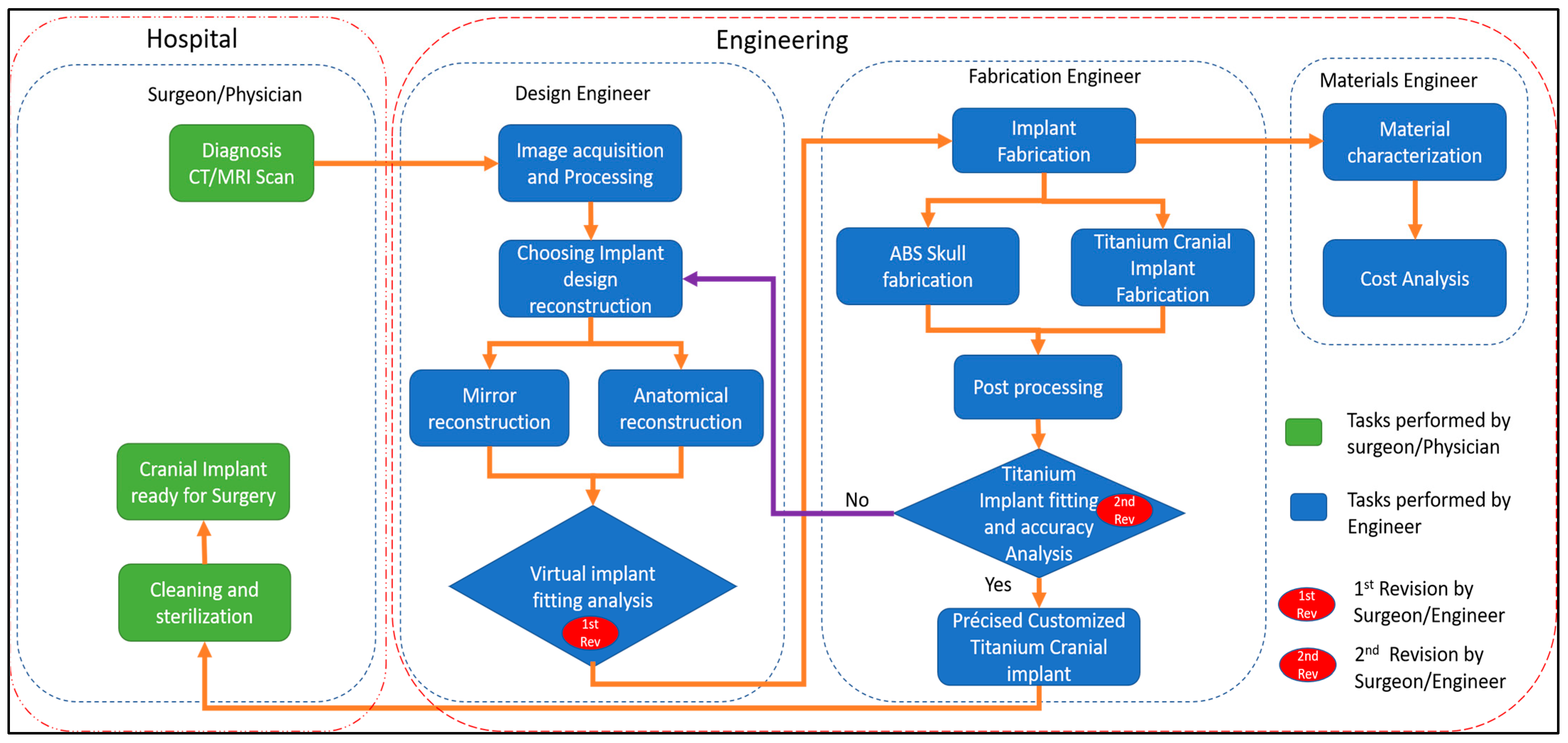
Source Image: mdpi.com
Download Image
Consider These Reactions Where M Represents A Generic Metal
Question: Consider these reactions, where M represents a generic metal. 1. 2. 3. 4. 2M (s)+6HCl (aq) 2MCl3 (aq)+3H2 ( g)HCl (g) HCl (aq)H2 ( g)+Cl2 ( g) 2HCl (g)MCl3 ( s) MCl3 (aq)ΔH1=−607.0 kJΔH2=−74.8 kJΔH3=−1845.0 kJΔH4=−233.0 kJ Use the given information to determine the enthalpy of the reaction 2M (s)+3Cl2 ( g) 2MCl3 ( s) ΔH= Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M_2 O(s) 2 M(s) + 1/2 O_2 (g) What is the standard change in Gibbs energy for the reaction Consider the following reaction: CaCO_3(s) to CaO(s)+CO_2(g).
Processes | Free Full-Text | Customized Cost-Effective Cranioplasty for Large Asymmetrical Defects
Consider these reactions, where M represents a generic metal. a. 2 M ( s ) + 6 HCl ( aq ) 2 MCl 3 ( aq ) + 3 H 2 ( g ) Δ H 1 = − 805.0 k J b. HCl ( g ) HCl ( aq ) Δ H 2 = − 74.8 k J c. H 2 ( g ) + Cl 2 ( g ) 2 HCl ( g ) Δ H 3 = − 1845.0 k J d. MCl 3 ( s ) MCl 3 ( aq ) Δ H 4 = − 484.0 k J Artworking with aluminium wire — artworkerprojects

Source Image: artworkerprojects.com
Download Image
If all the different kinds of metal music (power metal, deathcore, etc.) were people, what would be their personalities? – Quora Consider these reactions, where M represents a generic metal. a. 2 M ( s ) + 6 HCl ( aq ) 2 MCl 3 ( aq ) + 3 H 2 ( g ) Δ H 1 = − 805.0 k J b. HCl ( g ) HCl ( aq ) Δ H 2 = − 74.8 k J c. H 2 ( g ) + Cl 2 ( g ) 2 HCl ( g ) Δ H 3 = − 1845.0 k J d. MCl 3 ( s ) MCl 3 ( aq ) Δ H 4 = − 484.0 k J
Source Image: quora.com
Download Image
Consider these reactions where m represents a generic metal – YouTube Find stepby-step Chemistry solutions and your answer to the following textbook question: Consider these reactions, where M represents a generic metal. $$ \ce 2M (s) + 6HCl (aq)->2MCl3 (aq) + 3H2 (g), $\Delta H_1$ =−556.0 kJ $$ $$ \ce HCl (g)->HCl (aq) $\Delta H_2$ =−74.8 kJ $$ $$ \ce {H2 (g) + Cl2 (g)->2HCl (g) $\Delta H_3$ =−1845

Source Image: youtube.com
Download Image
Types of Chemical Reactions | Let’s Talk Science Chemistry Question Consider these reactions, where M represents a generic metal. 2M (s) + 6HCl (aq) \rightarrow → 2MCl _3 3 (aq) + 3H _2 2 (g) \hspace 1cm \Delta H_1 ΔH 1 = -600.0 kJ HCl (g) \rightarrow → HCl (aq) \hspace 3.9cm \Delta H_2 ΔH 2 = -74.8 kJ H _2 2 (g) + Cl _2 2 (g) \rightarrow → 2HCl (g) \hspace 2.9cm \Delta H_3 ΔH 3

Source Image: letstalkscience.ca
Download Image
How Schools Help Its Students to Find Their Passion – odmps blog Find stepby-step Chemistry solutions and your answer to the following textbook question: Consider these reactions, where M represents a generic metal. 2M(s)+6HCl(aq) 2MCl3(aq)+3H2(g) ΔH1 =−556.0 kJ HCl(g) HCl(aq) ΔH2 =−74.8 kJ H2(g)+Cl2(g) 2HCl(g) ΔH3 =−1845.0 kJ MCl3(s) MCl3(aq) ΔH4 =−342.0 kJ Use the given information to

Source Image: odmps.org
Download Image
Types of Materials | Let’s Talk Science Question: Consider these reactions, where M represents a generic metal. 1. 2. 3. 4. 2M (s)+6HCl (aq) 2MCl3 (aq)+3H2 ( g)HCl (g) HCl (aq)H2 ( g)+Cl2 ( g) 2HCl (g)MCl3 ( s) MCl3 (aq)ΔH1=−607.0 kJΔH2=−74.8 kJΔH3=−1845.0 kJΔH4=−233.0 kJ Use the given information to determine the enthalpy of the reaction 2M (s)+3Cl2 ( g) 2MCl3 ( s) ΔH=

Source Image: letstalkscience.ca
Download Image
Consider these reactions, where m represents a generic metal. 2m(s)+6hcl(aq)â¶2mcl3(aq)+3h2(g)δh1=â768.0 – brainly.com Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M_2 O(s) 2 M(s) + 1/2 O_2 (g) What is the standard change in Gibbs energy for the reaction Consider the following reaction: CaCO_3(s) to CaO(s)+CO_2(g).

Source Image: brainly.com
Download Image
If all the different kinds of metal music (power metal, deathcore, etc.) were people, what would be their personalities? – Quora
Consider these reactions, where m represents a generic metal. 2m(s)+6hcl(aq)â¶2mcl3(aq)+3h2(g)δh1=â768.0 – brainly.com Find stepby-step Chemistry solutions and your answer to the following textbook question: Consider these reactions, where M represents a generic metal 1.) $\ce 2M (s) + 6HCl (aq) \rarr 2MCl3 (aq) + 3H2 (g) \Delta H= -748.0 kJ $ 2.) $\ce HCl (g) \rarr HCl (aq) \Delta H= -74.8 kJ $ 3.) $\ce {H2 (g) + Cl2 (g) \rarr 2HCl (g) \Delta H= -1845 kJ
Types of Chemical Reactions | Let’s Talk Science Types of Materials | Let’s Talk Science Find stepby-step Chemistry solutions and your answer to the following textbook question: Consider these reactions, where M represents a generic metal. 2M(s)+6HCl(aq) 2MCl3(aq)+3H2(g) ΔH1 =−556.0 kJ HCl(g) HCl(aq) ΔH2 =−74.8 kJ H2(g)+Cl2(g) 2HCl(g) ΔH3 =−1845.0 kJ MCl3(s) MCl3(aq) ΔH4 =−342.0 kJ Use the given information to