Jan 26, 2023Therefore there are two types of molecular orbitals that can form from the overlap of two atomic s orbitals. The two types are illustrated in Figure 3.7A. 1 3.7 A. 1. The in-phase combination produces a lower energy bonding σs molecular orbital in which most of the electron density is directly between the nuclei.
covalent bonding and orbital overlaps | Lecture notes Chemistry | Docsity
The strength of a covalent bond is proportional to the amount of overlap between atomic orbitals; that is, the greater the overlap, the more stable the bond. An atom can use different combinations of atomic orbitals to maximize the overlap of orbitals used by bonded atoms. Figure 9.4.2 9.4. 2 shows an electron-pair bond formed by the overlap of

Source Image: sciencedirect.com
Download Image
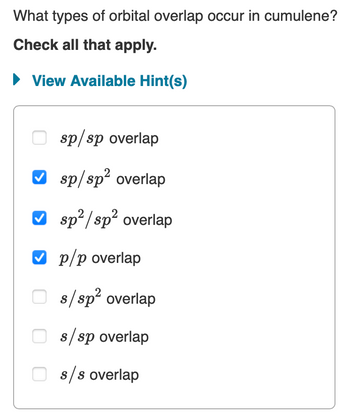
What types of orbital overlap occur in cumulene? Hybridization of Carbon: Carbon has two s and two p orbitals, a total of four valence electrons, which can hybridize to various extent:
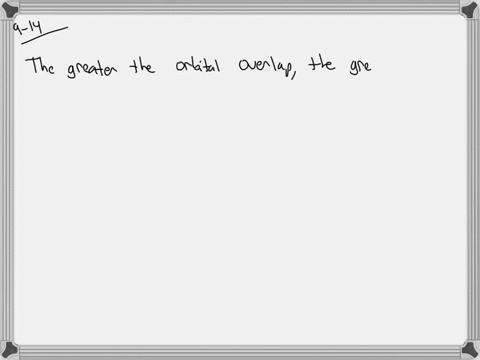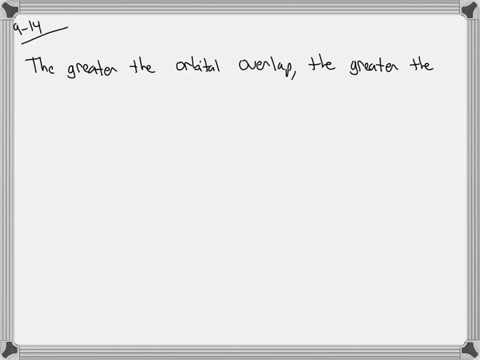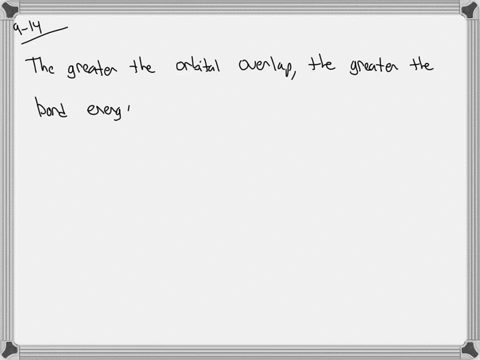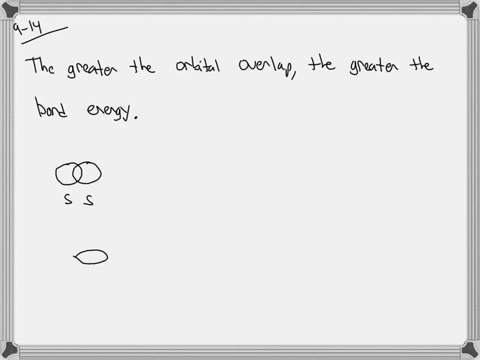
Source Image: numerade.com
Download Image
organic chemistry – Why does cyclopropane react with bromine? – Chemistry Stack Exchange Chemistry questions and answers. When two atoms form a single covalent bond, two What types of orbital overlap occur in cumulene? orbitals (one from each atom) overlap such that the Check all that apply. electron pair can be in both orbitals simultaneously. Double and triple bonds involve the sharing of View Available Hint (s) multiple electron

Source Image: numerade.com
Download Image
What Types Of Orbital Overlap Occur In Cumulene
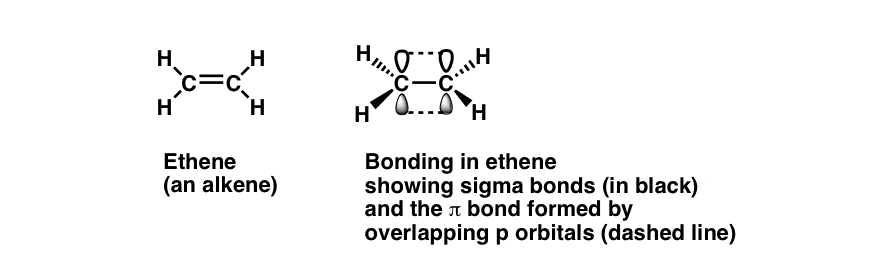
Chemistry questions and answers. When two atoms form a single covalent bond, two What types of orbital overlap occur in cumulene? orbitals (one from each atom) overlap such that the Check all that apply. electron pair can be in both orbitals simultaneously. Double and triple bonds involve the sharing of View Available Hint (s) multiple electron The orbitals overlap both above and below the plane of the molecule but form just one bonding orbital space. The C-C π π bond plus the hybridized C-C σ σ bond together form a double bond. Figure 10.3.1 10.3. 1: Bonding in Ethylene. (a) The σ σ -bonded framework is formed by the overlap of two sets of singly occupied carbon sp2 hybrid
SOLVED: What types of orbital overlap occur in cumulene? Check all that apply: sp2 / sp2 overlap sp / sp overlap sp2 overlap sp/sp2 overlap sp2 overlap p/p overlap π / π
What types of orbital overlap occur in cumulene? Verified Solution 10m This video solution was recommended by our tutors as helpful for the problem above. 222 Mark as completed Was this helpful? Previous problem Next problem 0:51m Watch next Master Hybridization Concept 1 with a bite sized video explanation from Jules Bruno Start learning Answered: What types of orbital overlap occur in… | bartleby
Source Image: bartleby.com
Download Image
Chiral Allenes And Chiral Axes – Master Organic Chemistry What types of orbital overlap occur in cumulene? Verified Solution 10m This video solution was recommended by our tutors as helpful for the problem above. 222 Mark as completed Was this helpful? Previous problem Next problem 0:51m Watch next Master Hybridization Concept 1 with a bite sized video explanation from Jules Bruno Start learning
Source Image: masterorganicchemistry.com
Download Image
covalent bonding and orbital overlaps | Lecture notes Chemistry | Docsity Jan 26, 2023Therefore there are two types of molecular orbitals that can form from the overlap of two atomic s orbitals. The two types are illustrated in Figure 3.7A. 1 3.7 A. 1. The in-phase combination produces a lower energy bonding σs molecular orbital in which most of the electron density is directly between the nuclei.

Source Image: docsity.com
Download Image
organic chemistry – Why does cyclopropane react with bromine? – Chemistry Stack Exchange What types of orbital overlap occur in cumulene? Hybridization of Carbon: Carbon has two s and two p orbitals, a total of four valence electrons, which can hybridize to various extent:
Source Image: chemistry.stackexchange.com
Download Image
There is free rotation about the carbon-carbon double bond of the alkenes. Is it true? – Quora What types of orbital overlap occur in cumulene? Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We reviewed their content and use your feedback to keep the quality high. Step 1. Cumulenes are compounds that contain consecutive multiple b
Source Image: quora.com
Download Image
computational organi.. Chemistry questions and answers. When two atoms form a single covalent bond, two What types of orbital overlap occur in cumulene? orbitals (one from each atom) overlap such that the Check all that apply. electron pair can be in both orbitals simultaneously. Double and triple bonds involve the sharing of View Available Hint (s) multiple electron

Source Image: yumpu.com
Download Image
MasteringChemistry (CHEM_261) Flashcards | Quizlet The orbitals overlap both above and below the plane of the molecule but form just one bonding orbital space. The C-C π π bond plus the hybridized C-C σ σ bond together form a double bond. Figure 10.3.1 10.3. 1: Bonding in Ethylene. (a) The σ σ -bonded framework is formed by the overlap of two sets of singly occupied carbon sp2 hybrid

Source Image: quizlet.com
Download Image
Chiral Allenes And Chiral Axes – Master Organic Chemistry
MasteringChemistry (CHEM_261) Flashcards | Quizlet The strength of a covalent bond is proportional to the amount of overlap between atomic orbitals; that is, the greater the overlap, the more stable the bond. An atom can use different combinations of atomic orbitals to maximize the overlap of orbitals used by bonded atoms. Figure 9.4.2 9.4. 2 shows an electron-pair bond formed by the overlap of
organic chemistry – Why does cyclopropane react with bromine? – Chemistry Stack Exchange computational organi.. What types of orbital overlap occur in cumulene? Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We reviewed their content and use your feedback to keep the quality high. Step 1. Cumulenes are compounds that contain consecutive multiple b